HTG EdgeSeq Precision Immuno-Oncology Panel
The next-generation sequencing (NGS)-based HTG EdgeSeq Precision Immuno-Oncology Panel is designed to measure the immune response both inside the tumor and the surrounding microenvironment. HTG’s quantitative nuclease protection assay does not require nucleic acid extraction and is automated using the HTG EdgeSeq processor. By leveraging the high sensitivity and dynamic range of NGS instrumentation, this powerful tool interrogates 1,392 genes from a single section of formalin-fixed, paraffin-embedded (FFPE) tissue, extracted RNA, or PAXgene samples.
For Research Use Only. Not for Use in Diagnostic Procedures.
Features and Benefits
- Small sample input: test specific tumor microenvironment (TME) regions or needle core biopsies
- 1,392 genes focused on tumor/immune interaction: see a broader view of the immune response in solid and liquid tumors
- FFPE and PAXgene samples: immunophenotype lymphocytes from both the tumor and peripheral blood
- Tumor subtyping: categorize tumors based on previously defined molecular expression and immune response phenotypes
- Simplified data analysis: analyze the result without a complicated pipeline
Research Applications
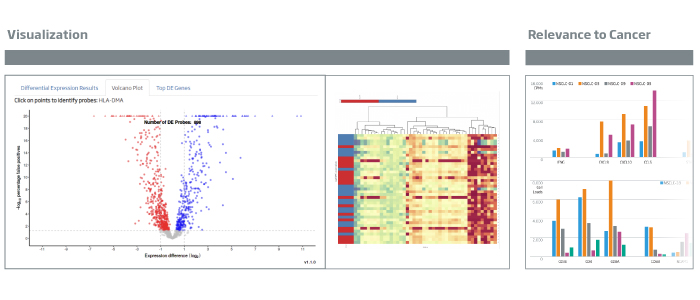
Tumor Inflammation
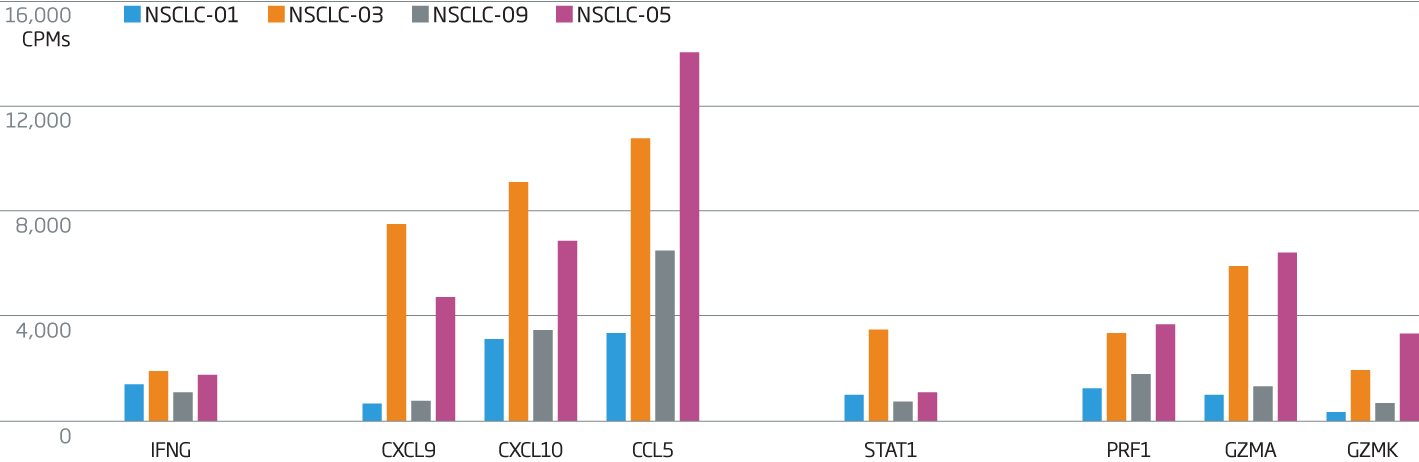
The breadth of the immune response in a tumor can be measured using the HTG EdgeSeq Precision Immuno-Oncology Panel. Relatively higher levels of IFNG (interferon γ), a key pro-inflammation cytokine, are measured in non-small cell lung cancer samples 3 and 5. IFNG production stimulates the production of T-cell attractant chemokines CXCL9, CXCL10, and CCL5, with a subsequent increase in production of cytolytic molecules such as perforin and granzymes.
Immunosuppression/EMT
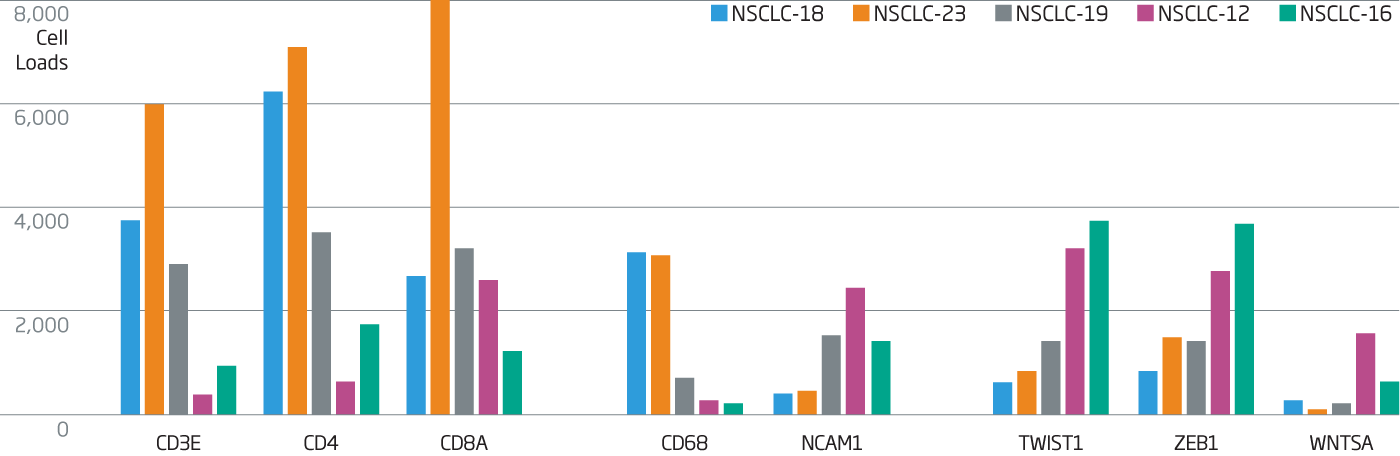
Immune loads of NSCLC tumors were assessed using T cell (CD3, CD4, and CD8) markers as well as CD68 (macrophages, myeloid cells). A range of expression was obtained indicating variable loads of immune cells.
Genes associated with WNT (WNT5A) and EMT drivers (ZEB1, TWIST1) are more highly expressed in tumors with lower immune loads. NK cells (NCAM1) are seen at higher levels in tumors with WNT and/or EMT activity, consistent with the literature report.
Other Applications
- TCGA tumor-subtyping
- Immunophenotyping TILs
- Immunoresistance pathways
- Tumor Inflammation
- Cytokine Profiling
- Immunosuppression phenotyping
- DNA repair mechanisms
- Drug target assessment
HTG EdgeSeq Reveal for Precision Immuno-Oncology Panel - Advancing Precision Medicine
HTG EdgeSeq Reveal is a powerful software product that streamlines analysis of biomarker data from samples analyzed with the
HTG EdgeSeq Precision Immuno-Oncology Panel on the HTG EdgeSeq system. HTG EdgeSeq Reveal enables characterization and visualization of immune response in the tumor environment which continues to be an important step in developing biomarker strategies to understand the body’s immune response in relation to cancer biology and potential response to mono- and combination therapies. The HTG EdgeSeq Reveal was designed to be a scalable, flexible data analysis product that will expand with new applications to support HTG’s growing translational research product portfolio.
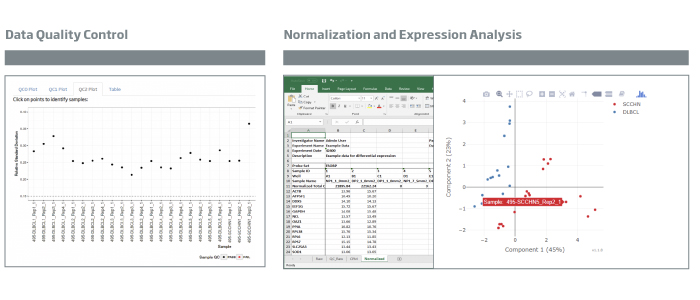
HTG’s new product, when coupled with the HTG EdgeSeq Precision Immuno-Oncology Panel, enables applications such as:
- Immunophenotyping of tumor infiltrating lymphocytes (TILs)
- Monitoring of immunotherapy response biomarkers
- Elucidation of immune-escape mechanisms known to drive disease progression
Together with the HTG EdgeSeq system, customers using the new HTG EdgeSeq Reveal product will have the ability to conduct molecular profiling using a wide variety of sample types, potentially enabling them to accelerate discovery, support translational applications, and determine potential biomarkers for development of companion diagnostics.
For more information and a product demonstration, contact your HTG representative.
Ordering Information
When placing an order, please specify the catalog number. The HTG EdgeSeq Precision Immuno-Oncology Panel is compatible with Illumina sequencers .
Kit Configurations for use with Illumina Sequencers
916-011-208 HTG EdgeSeq Precision I/O Panel (2X8)
916-011-008 HTG EdgeSeq Precision I/O Panel (4X8)
916-011-224 HTG EdgeSeq Precision I/O Panel (1X24)
916-011-024 HTG EdgeSeq Precision I/O Panel (4X24)
916-011-096 HTG EdgeSeq Precision I/O Panel (1X96)
Kit Configurations for use with Thermo Fisher Scientific Ion Torrent Sequencers
916-011-308 HTG EdgeSeq Precision I/O Panel (2x8)
916-011-108 HTG EdgeSeq Precision I/O Panel (4x8)
916-011-324 HTG EdgeSeq Precision I/O Panel (1x24)
916-011-124 HTG EdgeSeq Precision I/O Panel (4x24)
916-011-196 HTG EdgeSeq Precision I/O Panel (1x96)
For Research Use Only. Not for Use in Diagnostic Procedures.
Resources and Publications
Learn more about the HTG EdgeSeq Precision Immuno-Oncology Panel
Tech Note: Immunophenotyping Lymphocytes in Tumors
Download pdf 62KB
Tech Note: Measuring Immune Checkpoint Genes from FFPE Tissue
Download pdf 53KB
Tech Note: Assessing Tumor Inflammation
Download pdf 61KB
Tech Note: Epithelial Mesenchymal Transition in Immuno-Oncology
Download pdf 62KB
HTG EdgeSeq Precision Immuno-Oncology Panel Product Sheet
Download pdf 543KB
Annotated Gene List HTG EdgeSeq Precision Immuno-Oncology Panel
Download pdf 168KB
HTG EdgeSeq Precision Immuno-Oncology Panel Gene List
Download pdf 130KB
Publications
Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309)
View External Link
Phase II trial of neoadjuvant sitravatinib plus nivolumab in patients undergoing nephrectomy for locally advanced clear cell renal cell carcinoma
View External Link
Tumor Microenvironment and Its Clinicopathologic and Prognostic Association in Cutaneous and Noncutaneous Angiosarcomas
View External Link
Co-enrichment of CD8-positive T cells and macrophages is associated with clinical benefit of tislelizumab in solid tumors
View External Link
Understanding the immune microenvironment of pancreatic ductal adenocarcinoma patients. A transcriptomic study.
View External Link
25P - Prognostic classification of endometrial cancer according to transcriptomic-based immunophenotype
Download pdf 428KB
View External Link
Tumor Microenvironment and Its Clinicopathologic and Prognostic Association in Cutaneous and Noncutaneous Angiosarcomas
Download pdf 4.8MB
Understanding the immune microenvironment of pancreatic ductal adenocarcinoma patients. A transcriptomic study.
Download pdf 1.3MB
Prognostic classification of endometrial cancer
according to transcriptomic based immunophenotype
Download pdf 428KB
SABCS 2022: P3-05-09: vLAG3+ Tumor Infiltrating Lymphocytes Predict Outcome in Treatment Naïve Triple Negative Breast Carcinoma
Download pdf 874KB
View External Link
Gene Expression Profiling of CD23+ T(14;18)-negative follicular lymphoma demonstrates activation of the IL4/JAK/STAT6 pathway and a role in its pathogenesis
View External Link
Nivolumab in patients with relapsed or refractory peripheral T-cell lymphoma: modest activity and cases of hyperprogression
View External Link
Evaluation of the EdgeSeq Precision Immuno-Oncology Panel for Gene Expression Profiling From Clinical Formalin-Fixed Paraffin-Embedded Tumor Specimens
Download pdf 2.1MB
View External Link
CD8 T cells and macrophage abundances associated with clinical benefit of tislelizumab in various tumor types
View External Link
The combination of hyper-amplification and tumor mutational burden as a pan-cancer biomarker in patients treated with tislelizumab
View External Link
Genetic and phenotypic attributes of splenic marginal zone lymphoma
View External Link
360 Tumor-immune signatures associated with response or resistance to tislelizumab in patients with previously treated advanced hepatocellular carcinoma (HCC)
View External Link
High-dimensional and single-cell transcriptome analysis of tumor microenvironment in angioimmunoblastic T cell lymphoma AITL)
View External Link
CD73 expression defines immune, molecular, and clinicopathological subgroups of lung adenocarcinoma
Download pdf 2.2MB
HTG EdgeSeq technology offers a competitive alternative to RNA-Seq with equivalent performance and distinct advantages.
Download pdf 613KB
Sarcomatoid Renal Cell Carcinoma Demonstrates an Immunosuppressive Tumor Microenvironment - Implication for Therapeutic Benefit in the Immunotherapy Era
Download pptx 4.4MB
Next Generation Sequencing and Functional Pathway Analysis to Understand the Mechanism of Action of Copper-Tolfenamic Acid Against Pancreatic Cancer.
View External Link
Neutrophil content predicts lymphocyte depletion and anti-PD1 treatment failure in NSCLC
Download pdf 16.7MB
Genetic associations of T cell cancer immune response with tumor aggressiveness in localized prostate cancer patients and disease reclassification in an active surveillance cohort.
Download pdf 1.3MB
Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial
Download pdf 1.0MB
Dynamic change of immune-related gene expression status during chemoradiotherapy in locally advanced esophageal cancer
View External Link
Learn More
For further information about the HTG EdgeSeq Precision Immuno-Oncology Panel, please fill out the registration form below or call us at (877) 507-3259.
Page last updated June 29, 2022